Episode Transcript
Interviewer: Stopping cancer before it even starts. We'll talk about that next on The Scope.
Announcer: Examining the latest research and telling you about the latest breakthroughs. The Science and Research Show is on The Scope.
Interviewer: I'm talking with Doctor Deb Neklason, Huntsman Cancer Institute Investigator and Program Director of the Utah Genome Project. Dr. Neklason, congratulations on your recent JAMMA publication. What did the results of your clinical trials show?
Dr. Neklason: This clinical trial showed that we were able to treat individuals that had a hereditary predisposition to gastrointestinal cancers. We were able to reduce the polyps in their small intestine with about a 75% response rate.
Interviewer: 75%. I mean, that's a lot.
Dr. Neklason: It was a huge response and they've never seen anything like that.
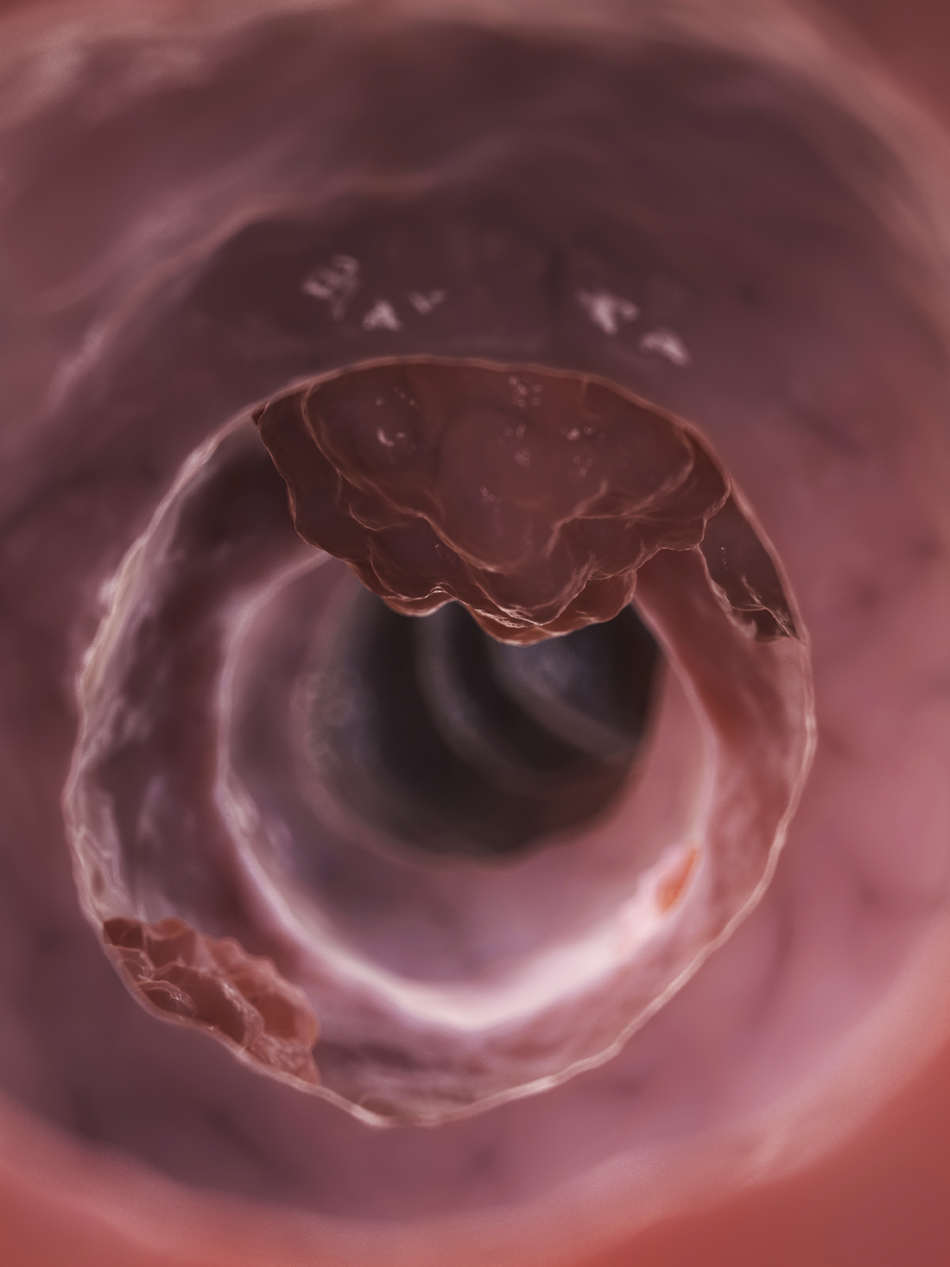
Interviewer: And what is a polyp?
Dr. Neklason: So the polyps are precancerous lesions that are their duodenum, which is part of the small intestine just after the stomach. These individuals have about a 10% to 12% risk of developing duodenal cancer. If we can find a way to actually drive these precancerous polyps away with a drug instead of having to go in and cut it out every time, it's just a huge proof of principle, a huge success.
Interviewer: And do the people that took part in this trial have a certain type of colon cancer?
Dr. Neklason: Yes, so this is a fairly rare genetic condition. It's about 1 in 10,000 individuals and it come about from a genetic change in a gene called the APC or adenomatous polyposis coli gene. These individuals develop hundreds to thousands of polyps in their colon. They have 100% risk of developing colon cancer if it's not managed clinically and by that they usually end up having a colectomy where their colon is removed and then reattached.
That then eliminates most of that risk of colon cancer in those individuals, but then they still have the risk of other cancers, namely this duodenal cancer. That is very much an unmet need for these individuals. They run the risk of still developing cancer and you can't really take your small intestine out because it's essential for nutrition and digestion and you don't do very well without your small intestine.
Interviewer: How did you arrive at this drug therapy? What made you choose this combination?
Dr. Neklason: So the drug combination we used is Sulindac, which is a non-steroidal anti-inflammatory, kind of like aspirin or ibuprofen. It's used for arthritis but it inhibits a really important gene that's overexpressed in the colon tissue and the duodenal tissue, especially as they advance to become polyps and cancer.
This drug, Sulindac, worked really well to drive regression of colon polyps but it didn't do anything to the duodenal polyps. The thought was that this COX-2 protein was expressed at much higher levels and they couldn't use that drug at high levels. Through our work here at Huntsman Cancer Institute and University of Utah we, as well as others throughout the country, started to pick apart the pathway that turns on this gene. We know that APC, the gene that's altered in these individuals, is important in driving up expression of that and we also discovered that there is a feedback from epidermal growth factor receptor, which is eGFR There's a lot of new drugs that have been developed against eGFR because this is overexpressed in a whole bunch of cancers.
We choose to use a small molecule inhibitor of eGFR called erlotinib, and our thought was if we can hit two segments of the pathway with these two drugs, maybe we can have an effect in the duodenum, and indeed, we were successful with that.
Interviewer: Do you have plans to track them further out? What are some of the next steps with this trial?
Dr. Neklason: There are some really important end points that we need to figure out. One of the important questions that you alluded to is what happens when you take them off drug? Do the polyps come right back? Or we talk about the durability of the response. Is it repressed for maybe a year out and would the design need to be where you cycle them, put them on for six months, off for a year, on for six months, or what would it look like in that way?
Probably even more important is to follow these individuals long-term and actually show a different clinical outcome. What I mean by that is do we prevent them from having to undergo surgery? People that are treated with the drug, do they undergo less surgeries than people that are on placebo? Or even do we prevent cancers in these individuals? Those end up being five, ten, fifteen year studies to be able get a good solid result that you understand.
Interviewer: So here you are testing this potentially new drug therapy in clinical trials and these families who are stricken with colon cancer, and this is really where the whole project started. In a way this is kind of bringing work here at the University of Utah full circle.
Dr. Neklason: This goes back to the late 1980s. There is a team of researchers, including my mentors, that discovered the APC gene. They have gone on the . . . Randy Burt, the clinician who managed these patients has just retired, but he is a legacy in and of himself for treating and managing people with familial adenomatous polyposis and other polyposis conditions.
It's very exciting because we've identified the gene. Over the years we've studied how does the gene work. We've studied the patients, how does the disease progress in them? We're finally at a point where we can precisely understand what's going on in those cells and prevent the disease. This whole idea of precision medicine, we like to think of this as precision prevention.
Interviewer: One of the interesting things that you actually mentioned is that the mutation that causes FAP, this inherited cancer, is in the APC gene and that gene is mutated in sporadic cancers as well. Do you think this therapy could have implications for other colon cancers too?
Dr. Neklason: I think that what we call the proof of principle, I think the fact that we know that we can target these pathways with these drugs will enable us to make better design of treatments down the road. The APC gene is altered in a very early step of cancer progression.
There are some really exciting analogies with colon cancer where it's known that aspirin, regular aspirin, can reduce the risk of colon cancer for people that are at pretty high risk, so that's a drug a little bit like Sulindac. It's quite possible that that knowledge can be used in the prevention and potentially even the treatment because the eGFR is known to be overexpressed in a lot of cancers, so just understanding how we can manipulate that pathway and as we understand cancers better, colon cancers, even lung cancers have a lot of eGFR expression, that just understanding that we can actually get the drug into the body where it needs to go, do what it needs to do, can be applied more broadly.
Announcer: Interesting, informative and all in the name of better health. This is The Scope Health Sciences Radio.